Introduction
Phosphate Rock (PR) has long been an important agriculture-based commodity. Most soils are phosphate deficient, and with the majority of 3rd world countries relying on cereals which require phosphate rich environments to thrive, the need for further phosphate exploration and exploitation will continue to grow well past 2050. With the ability of phosphates to sequester heavy elements during crystallization, a recent push has been made to also develop key deposits as rare Earth element (REE) ores. While sources vary, phosphate describes the minerals: hydroxylapatite (HAp), fluorapatite (FAp), francolite and carbonate-hydroxylapatite; the latter being a disordered form of hydroxyapatite occurring most commonly in aqueous depositional environments derived from the dissolution and reprecipitation of animal bone.
Phosphate Rock Mineralogy

Phosphate rock describes an imprecise series of minerals, all belonging to or variations of the apatite group of minerals (Figure 1). As a whole the general chemistry is A5(XO4)3(OH,F,Cl), where A is generally Ca and X is P, however the mineral group as a whole can accommodate over half the periodic table amongst the two sites. Furthermore, in the case of francolite and carbonate-hydroxylapatite, CO3 can substitute into the PO4 site at a ratio of no more than 1:4 respectively. Chlorapatite, is rare and of no economic importance as the Cl removal erodes processing plant parts leading to costly maintenance. Ideally, apatite has a hexagonal symmetry with a space group of P63/m and cell dimension ranges of a = 9.39 (F) – 9.42 (OH) Å and c = 6.88 Å. The range of values along the a-axis is due to the order-disorder effects of the 0,0,z site between the F and OH variants. A list of phosphate rock minerals, chemistries and a-axis cell dimensions is given in Table 1.
| Variety | Formula | Unit Cell a-value (Å) |
|---|---|---|
| Fluorapatite | Ca5(PO4)3F | 9.370 |
| Hydroxylapatite | Ca5(PO4)3(OH) | 9.420 |
| Carbonate-hydroxylapatite | Ca5(PO4,CO3)3(OH) | 9.420 |
| Francolite | Ca5-x-yNaxMgy(PO4)3-z(CO3)zFF0.2z | 9.320-9.369 |
| Values derived from VanKauwenbergh (2010) | ||
Occurrences and Mineral Deposits
Phosphate rock occurs either as sedimentary or igneous deposits with sedimentary sources comprising roughly 80% of the world’s production. Igneous sources are more difficult due to the high degree of crystallinity, making the ore less soluble for processing. Sedimentary phosphate rock sources are usually described as loose unconsolidated to lightly cemented deposits consisting of sand to cobble size grains. Sedimentary deposits can usually be mined as an open pit, reducing the production costs. Phosphate rock often occurs with quartz and feldspars, with economically viable deposits consisting of at least 8-18% P2O5 and the ability to beneficiate to a maximum of 42%P2O5.
Sedimentary deposits occur throughout the geologic timescale, exhibiting a wide variety of chemical compositions and textures. The most ideal deposits occur as one or more thick, unconsolidated beds of
high-grade ore which has a uniform granular texture. Such deposits also exhibit little overburden and minimal structural deformations.
Historically, insular deposits have been an important source for phosphate rock for over 100 years. Such deposits are derived from oceanic islands. Due to extensive mining and exploitation over this long history, such deposits (e.g. Nauru, Christmas Island and Ocean Island) are entirely depleted, or those that exist offer only a short-lived mining potential.
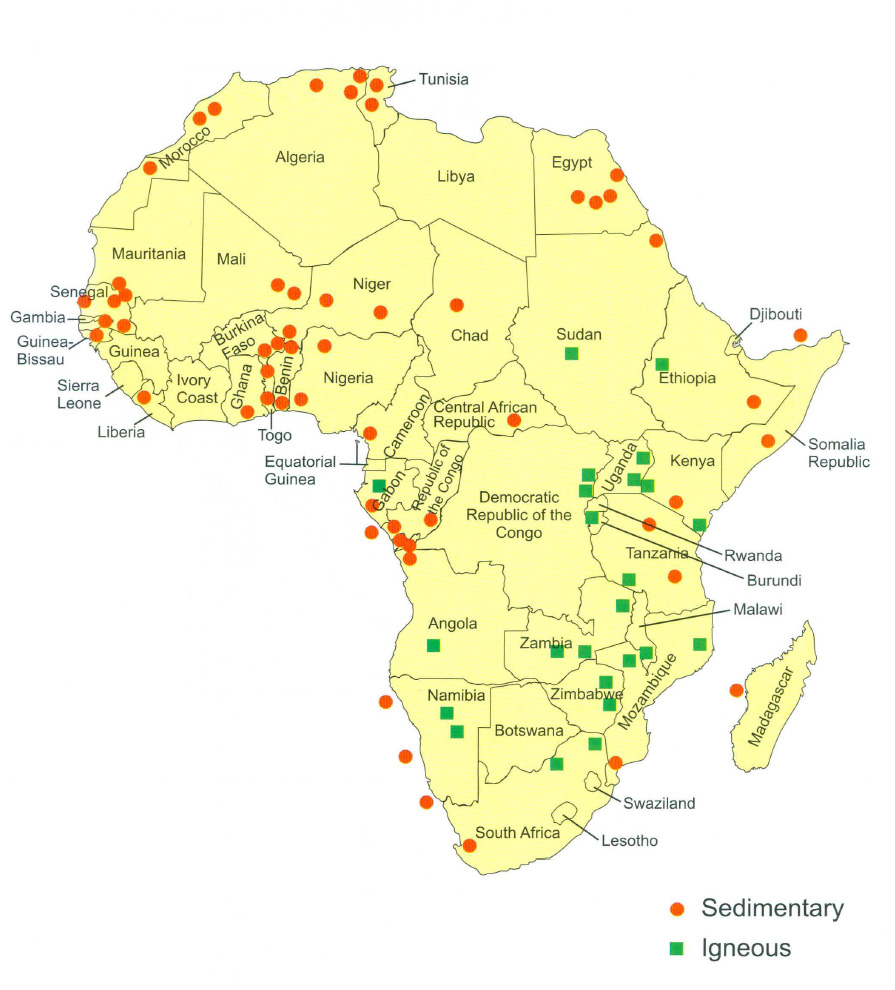
The majority of active economic phosphate deposits are located in Africa and the Middle East. According to the USGS Mineral Commodities Summary (2019), China is the largest producer of phosphate rock, at nearly 144 million metric tons gross production in 2018, with other significant deposits located in Morocco (who hosts the largest reserves estimated at 50 billion metric tons), Jordan, Egypt, Togo, Benin, throughout sub-Sahara Africa, North Carolina, Idaho and Russia. Currently, the most economically important deposits reside in Africa (Figure 2). However, new deposits are consistently being discovered and developed.
Phosphate Fertilizer Production
The single highest demand for phosphate rock is the fertilizer industry. By reacting phosphate rock with either sulfuric or nitric acid, a variety of phosphate fertilizer products can be made. These include mono- and di-ammonium phosphate (MAP & DAP), Single Super Phosphate (SSP), Triple Super Phosphate (TSP), Partially Acidulated Phosphate Rock (PAPR) and Direct Application Phosphate Rock (DAPR). Current market values drive a particular fertilizer product’s production. Few deposits are viable for DAPR as this requires a highly soluble phosphate rock. Of particular note is the Minjingu Phosphate deposit in Minjingu, Tanzania. This site produces the most appropriate phosphate rock for DAPR applications.
Conclusion
Phosphate rock plays a critical role in the nutrient cycle of many soils. Of significant importance to arid regions, phosphate fertilizers are necessary for the growth of staple crops, such as corn or cereals. Such rock is mainly obtained through the open-pit mining of sedimentary deposits containing one or more ideally unconsolidated, chemically homogenous layers. While there has been speculation that phosphate is a resource that could be in danger, current resource and reserve estimates along with the discovery and exploitation of new deposits suggest that world production will continue for at least the next 250+ years. Furthermore, continued research into the exploitation of phosphate rock deposits as an ore source for REEs drives the exploration of deposits which may not be suitable for fertilizer applications. Currently, China is leading the world in production and exploitation of deposits, overtaking Morocco in recent years.
Further Reading
- VanKauwenbergh, S.J. (2006) “Fertilizer Raw Material Resources of Africa”. International Fertilizer Development Center. PDF
- VanKauwenbergh, S.J. (2010) “World Phosphate Rock Reserves and Resources”. International Fertilizer Development Center. General Publication IFDC-G-1. www.ifdc.org.
- Jasinski, S. (2019) “Phosphate Rock Statistics and Information: Mineral Commodities Survey”. USGS. PDF
- Lafuente B, Downs R T, Yang H, Stone N (2015) The power of databases: the RRUFF project. In: Highlights in Mineralogical Crystallography, T Armbruster and R M Danisi, eds. Berlin, Germany, W. De Gruyter, pp 1-30. www.rruff.info.